Thermodynamic Studies of Systems Containing Amino Acids or Derivatives with Electrolytes
The study of ion specific effects on the aqueous solubility of amino acids and proteins is crucial for the development of many areas of biochemistry and biotechnology. However, direct study of protein-electrolyte interactions is difficult and it is therefore useful to investigate the interaction of model compounds such as amino acids, peptides, and their derivatives. In this research area, the effect of ions with biological relevance on the aqueous solubility of amino acids or derivatives is studied by performing experimental solubility measurements and molecular dynamics simulations. Partial molar volumes are also evaluated, aiming to provide further insights into the interactions of these biomolecules in electrolyte solutions.


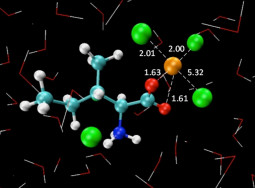
L.I.N. Tomé, C.S.R. Sousa, J.R.B. Gomes, O. Ferreira, J.A.P. Coutinho, S.P. Pinho. Understanding the cation specific effects on the aqueous solubility of amino acids: From mono to polyvalent cations. RSC Advances, 5, 15024-15034, 2015
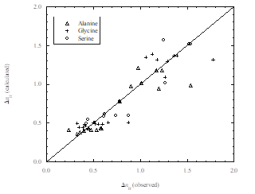
E. Javornik, O. Ferreira, S.P. Pinho. Volumetric interactions of a series of _-amino acids in aqueous magnesium chloride solutions at 278.15, 288.15, 298.15, and 308.15 K. Monatshefte fur Chemie, 146, 1419-1431, 2015
M.F. Vilarinho, I.C. Fernandes, S.P. Pinho. Water activity in aqueous amino acid solutions containing ammonium sulfate at 298.2 K. Journal of Chemical and Engineering Data, 59, 1802-1807, 2014
P.C. Mota, O. Ferreira, L. Hnědkovský, S.P. Pinho, I. Cibulka. Partial molar volumes of l-serine and l-threonine in aqueous ammonium sulfate solutions at (278.15, 288.15, 298.15, and 308.15) k. Journal of Solution Chemistry, 43, 283-297, 2014
M.A.R. Martins, O. Ferreira, L. Hnědkovský, I. Cibulka, S.P. Pinho. Partial molar volumes of glycine and dl-alanine in aqueous ammonium sulfate solutions at 278.15, 288.15, 298.15 and 308.15 K. Journal of Solution Chemistry, 43, 972-988, 2014
L.I.N. Tomé, S.P. Pinho, M. Jorge, J.R.B. Gomes, J.A.P. Coutinho. Salting-in with a salting-out agent: Explaining the cation specific effects on the aqueous solubility of amino acids. Journal of Physical Chemistry B, 117, 6116-6128, 2013




